Background: Multiple myeloma (MM) is an incurable, heterogeneous neoplasm of clonal plasma cells preceded by the precursor conditions monoclonal gammopathy of undetermined significance (MGUS) and asymptomatic multiple myeloma (AMM). Features of metabolic syndrome including obesity and diabetes mellitus (DM) have been linked to MM development and mortality. A study of serum metabolomics in patients with clinical MM revealed alterations in the citrate cycle, pyrimidine, purine, and amino acid metabolism (Chanukuppa V, et al, 2019). However, the role of dysregulated metabolism in progression of monoclonal gammopathy across the disease spectrum requires further investigation. We performed a pilot study to compare metabolic profiles in patients with MGUS and AMM.
Methods: Clinical data and peripheral blood plasma samples were collected on 20 patients with non-immunoglobulin M MGUS and 20 patients with AMM. Global metabolomic studies by liquid chromatography (LC) tandem mass spectrometry (MS) were performed on plasma using a SCIEX TripleTOF 6600+ mass spectrometer. Each sample was run on Hypercarb and Reverse phase LC columns in both positive and negative MS modes. Standard targeted and untargeted metabolomic platforms were used with assessment of changing metabolites between the two groups in a one-to-one comparison. Correlation analysis and differential expression analysis were completed and top changing metabolites determined based on ranking of log fold change and p value cutoff (at least <0.05). Pathway enrichment data was generated with advanced data analysis.
Results: Median age was 70 and 67 years in the MGUS and AMM groups, respectively. 65% of patients in the AMM group were male versus (vs) 40% in the MGUS group. Black individuals represented 15% of MGUS and 30% of AMM patients. Mean serum monoclonal (M) protein was higher in AMM patients (0.87 g/dL vs 0.28 g/dL, p=0.009); mean bone marrow clonal plasma cells in AMM was 17% (range 10-40%). Three AMM patients had high risk cytogenetics inclusive of chromosome 1 abnormalities. We observed that more individuals in the AMM group had a diagnosis of DM (35% vs 15%), were on hyperlipidemia (HLD) medications (80% vs 45%) and were on ≥3 hypertension (HTN) medications (35% vs 20%). Increasing weight and hemoglobin a1c values were seen with rise in serum M protein in the combined groups.
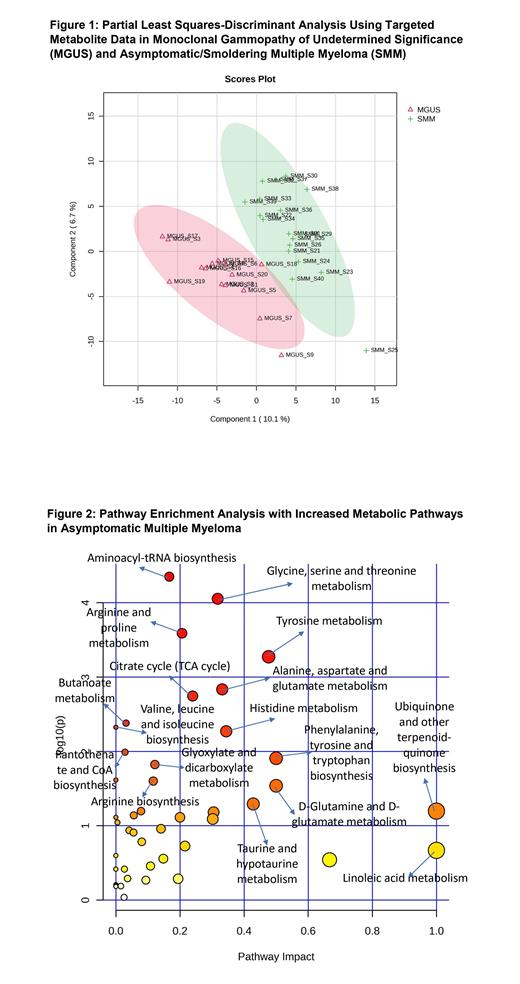
For global metabolomics, 2,513 metabolites were annotated from targeted and untargeted peak curation and results underwent data normalization and standardization with exclusion of outliers from the data set. Using targeted data, Partial Least Squares-Discriminant Analysis showed separation of the MGUS and AMM groups (Figure 1). There were 8 significantly increased metabolites (dihydrouracil, quinate, melatonin, azelate, phosphonoacetate, malate, L-alanine, and lysine) and 9 significantly decreased metabolites (diethanolamine, xanthosine, nervonate, quinolinate, L-dopa, ethylmalonate, butanoate, carnosine, and thyroxine) in AMM when compared to MGUS. Pathway enrichment analysis revealed several pathways increased in AMM (Figure 2), including D-glutamine and D-glutamate metabolism and the citrate cycle. Pathways decreased in AMM included purine, pyrimidine, and glutathione metabolism.
Conclusions: In our study, patients with AMM were more likely to demonstrate features of metabolic syndrome, including higher rate of DM and need for HTN and HLD medications. Additionally, a trend of higher weight and hemoglobin a1c values occurred as serum M protein increased. Global metabolomics identified unique metabolites that differed in AMM relative to MGUS patients. The unsaturated fatty acid nervonate, decreased in AMM in our study, was previously shown to have potential preventive effects on obesity-related metabolic disorders (Oda E, et al, 2005). Our findings in these precursor cohorts are concordant with prior serum metabolomics in MM showing alterations in amino acid metabolism including glutamine (Chanukuppa V, et al, 2019), with glutaminase inhibitors currently under study in relapsed/refractory MM (Bolzoni M, et al, 2016). Additional investigation of metabolic profile in larger groups across the spectrum of monoclonal gammopathy will help further elucidate the role of this dysregulated metabolism in myelomagenesis and could lead to preventative interventions targeting the associated metabolic syndrome earlier in disease course.
Disclosures
Browning:Janssen: Research Funding. Neparidze:GSK: Research Funding; Janssen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal